Engineering and Applied Science Letters
ISSN: 2617-9709 (Online) 2617-9695 (Print)
DOI: 10.30538/psrp-easl2018.0008
Biological Synthesis and Characterization of Chromium (iii) Oxide Nanoparticles
Zaheer Ahmad, Aisha Shamim, Sajid Mahmood\(^{1}\), Tariq Mahmood, Farman Ullah Khan
Department of Chemistry, University of Wah, Wah Cantt, Pakistan.; (Z.A, A.S & F.U.K)
Department of Chemistry, Division of Science & Technology,University of Education, Township Campus, Lahore, Pakistan.; (S.M)
Nano Science and Technology Department, National Centre for Physics, Quaid-e-Azam University, Islamabad 45320, Pakistan.;(T.M)
\(^{1}\)Corresponding Author; drsajidue@gmail.com
Abstract
Index Terms:
1. Introduction
Nanotechnology is an escalating field in recent science [1] that controls matter which is extremely small (< 100nm). Idea of Nanotechnology was proposed by Richard Feynman [2] and Professor Norio Taniguchi coined this term in 1974. Nanotechnology is the segregation, organization, and strengthening of materials. Nanoparticles are ultrafine particles [3] with exceptional physico-chemical characteristics which are the result of quantum confinement effect [4]. In particles of the size less than 70nm Vander waals force becomes significant. Gecko can climb the walls due to nanosized hairs on its limbs. Gold NPs change their ability to reflect light and Al NPs are extremely reactive when their size is less than 20nm. Nanoparticles are fascinating because of their change of properties when they are very small [5]. Concept of Nanoparticles is not new, they have been in use since 4th century A.D, for example Lycurgus cup [6]. Nanoparticles have various applications in catalysis, electronic devices, dyes and pigments [6, 7].
Physical, chemical, and biological methods are commonly used for the synthesis of nanoparticles. Physical methods are free from contaminants but are uneconomical because of formation of plentiful waste. Physical methods used for the synthesis of nanoparticles are ball-milling, ablation, and pyrolysis. Various chemical methods used for the synthesis of nanoparticles are sol-gel method, microemulsion, hydrothermal, and chemical vapor deposition. Biological methods are clean and environment friendly methods. Various biological entities like fungi, bacteria, and plants are used in this method. This method involves enzymes, proteins, and NADH reductase coenzyme [8].
Biological methods using microorganisms are clean, nonhazardous, and eco friendly. These are fast and are carried out at ordinary conditions. Microorganisms are adapted to harsh conditions by using survival strategies such as efflux system, extracellular precipitation and chemical detoxification. Reduction, biosorption, and bioaccumulation are important mechanisms for biosynthesis [9, 10].
Chromium (iii) oxide nanoparticles are one of the unique transition metal compounds [11] that have won much attention of researchers because of their extensive use in science and technology [12]. The \(Cr_2O_3\) nanoparticles are manufactured by an aqueous precipitation method using chromic sulphate as a template and ammonia as a precipitating agent. Gibot and Vidal synthesized spherical \(Cr_2O_3\) nanoparticles by thermal decomposition of \(Cr (NO_3)_3.9H_2O\) [13]. Pei et al. synthesized \(Cr_2O_3\) nanoparticles by using \(CrO_3\) and \(C_2H_5OH.\) Jaswal et al. synthesized \(Cr_2O_3\) NPs by precipitation method using chromic sulphate and ammonia. This method is of low cost and environment friendly [14].
\(Cr_2O_3\) NPs are fabricated by reducing potassium dichromate solution with Tridaxprocumbens leaf extract [15] and Allium sativum [16]. Pure and uniform sized \(Cr_2O_3\) NPs with cubic morphology were synthesized by a green chemistry method using Callistemon viminalis flowers extract [17]. Chromium oxide NPs were synthesized by mixing potassium dichromate solution with pumpkin leaves extract. The solution was dried at 70°C for 6 hours and then calcined at 650°C [18]. The use of bioorganisms is ecofriendly approach with natural reducing and capping agents [19]. Chromium oxide NPs find wide range of applications in colorants, catalysts, coatings [20], green pigment, solar energy collectors, and liquid crystal displays [21].
2. Experimental
In present study we synthesize \(Cr_2O_3\) NPs by using biological method. This study is carried out at Nanoscience and Technology Department, National Centre for Physics, Quaid-e-Azam University, Islamabad and Department of Chemistry, University of Wah, Wah-Cantt. These nanoparticles are characterized by using X-ray diffraction, UV-Visible spectroscopy, Scanning Electron Microscopy and Electron Dispersive X-ray Spectroscopy. During this work all chemicals are purchased from local market of Sigma-Aldrich. These were AR-Grade and there was no need of further purification. We used deionized water throughout the experiment.2.1. Biological Synthesis
In this method first of all salt solution is prepared by dissolving 3-4g of salt (\(Cr_2 (SO_4)_3\)) in deionized water and mixed with 2g crushed powder of Aspargillusniger and stirred for 30 minutes. Then the resultant material ia placed in dark for 3 days. After 3 days we filtered the solution. The filtrate is characterized by UV-Visible for finding size and concentration of nanoparticles. The filtrate is then dried and calcined in furnace at 550°C for 3 hours. Now the material is grinded and analyzed by XRD, SEM and EDX.We analyzed nanoparticles by using XRD model D8 ADVANCE BRUKER X-Source Copper/(anode). UV-Vis is performed on UV-Vis Spectrometer Perkin Elmer, Lambda 25. Both instruments are placed at Nanoscience and Technology Department, Quaid-e-Azam University, Islamabad. The Scherrer formula is used for finding size. The formed NPs are characterized by XRD and their results arenoted in nanometer. The synthesized NPs are also characterized by SEM performed on SEM, TESCAN, VEGA3 placed at Advanced Energy and Material lab NUST. The SEM study is carried out to find size and morphology of nanoparticles. The EDX is done on EDX Oxford placed at Fracture Mechanics and Fatigue Lab, Mechanical Engineering Department, UET Taxila. The EDX is used to find elemental composition and purity of samples.
3. Results and discussions
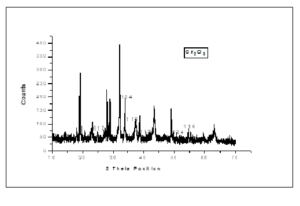
We characterized NPs by XRD. The XRD is used to find size of particles and crystallinity. The Scherrer formula is used to find crystallite size of NPs. The XRD analysis of biologically synthesized Cr2O3 nanoparticles is described Figure 1.Figure 1. XRD Spectrum of \(Cr_2O_3\) NPs
Table 1. XRD Data of \(Cr_2O_3\) NPs
| PEAKS | 2θ POSITION | hkl VALUES | d-SPACING |
|---|---|---|---|
| 1 | 24.720 | 012 | 3.5986 |
| 2 | 33.912 | 104 | 2.6412 |
| 3 | 36.530 | 110 | 2.4578 |
| 4 | 41.865 | 113 | 2.1560 |
| 5 | 50.696 | 024 | 1.7992 |
| 6 | 55.381 | 116 | 1.6576 |
| 7 | 65.760 | 300 | 1.4189 |
Figure 2. SEM of \(Cr_2O_3\) NPS (500nm)
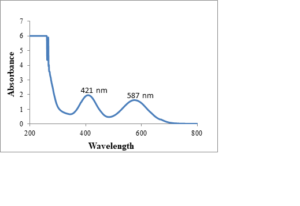
Figure 3. UV-Vis Spectrum of \(Cr_2O_3\) NPs
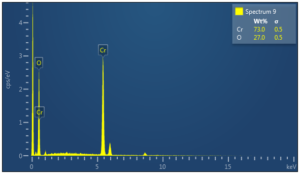
Figure 4. EDX Spectrum of \(Cr_2O_3\) NPs
4. Conclusion
The present study shows that Chromium oxide NPs were successfully synthesized by biological method using fungal extract. The XRD study shows that the size of biologically synthesized \(Cr_2O_3\) NPs is 36nm. The SEM study shows that \(Cr_2O_3\) NPs are hexagonal. The EDX shows that synthesized nanoparticles are pure and there is only trace of impurities present in the samples. Chromium oxide NPs have applications in the stropping of knives, glasses, inks, paints and precursor to the magnetic pigment.Acknowledgement
We acknowledge the provision of services of NS & TD, NCP, QAU Islamabad.Competing Interests
The authors do not have any competing interests in the manuscript.References
- Panigrahi, T. (2013). Synthesis and Characterization of Silver Nanoparticles using leaf extract of Azadirachta indica (Doctoral dissertation).[Google Scholor]
- Ghiuţă, I., Cristea, D., & Munteanu, D. (2017). Synthesis Methods of Metallic Nanoparticles- An Overview. Bulletin of the Transilvania University of Brasov. Engineering Sciences. Series I, 10(2), 133-140.[Google Scholor]
- Bhatia, S. (2016). Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural polymer drug delivery systems (pp. 33-93). Springer, Cham. [Google Scholor]
- Kumar, U. (2011). Biosynthesis of metal/metal-oxide nanoparticles and measurement of their physical, biophysical properties.[Google Scholor]
- Trybula, W., Fazarro, D. E., & Kornegay, A. (2009). The emergence of nanotechnology: Establishing the new 21st century workforce. Online Journal for Workforce Education and Development, 3(4), 6.[Google Scholor]
- Ban, I., Stergar, J., Drofenik, M., Ferk, G., & Makovec, D. (2014). Synthesis of chromium-nickel nanoparticles prepared by a microemulsion method and mechanical milling. Acta Chimica Slovenica, 60(4), 750-755. [Google Scholor]
- Li, X., Xu, H., Chen, Z. S., & Chen, G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. Journal of Nanomaterials, 2011. [Google Scholor]
- Kumar, H.K., Venkatesh, N., Bhowmik, H., & Kuila, A. (2018). Metallic Nanoparticles: A Review. Biomed. JSci & Tech, A. (2).
- Chokriwal, A., Sharma, M. M., & Singh, A. (2014). Biological synthesis of nanoparticles using bacteria and their applications. American Journal of PharmTech Research, 4(6), 38-61.[Google Scholor]
- Thakkar, K. N., Mhatre, S. S., & Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 6(2), 257-262. [Google Scholor]
- Farzaneh, F. (2011). Synthesis and characterization of Cr2O3 nanoparticles with triethanolamine in water under microwave irradiation. Journal of Sciences, Islamic Republic of Iran, 22(4), 329-333. [Google Scholor]
- Zhang, X. F., Liu, Z. G., Shen, W., Gurunathan, S. (2016). Silver Nanoparticles: Synthesis, Characteristics, Properties, Applications, and Therapeutic Approaches. International J. MolSci., 17(9), 1534.
- Jaswal, V. S., Arora, A. K., Singh, J., Kinger, M., & Gupta, V. D. (2014). Synthesis and characterization of chromium oxide nanoparticles. Oriental Journal of Chemistry, 30(2), 559-566.[Google Scholor]
- Iqbal, T., Tufail, S., & Ghazal, S. (2017). Synthesis of Silver, Chromium, Manganese, Tin and Iron Nano Particles by Different Techniques. International Journal of Nanoscience and Nanotechnology, 13(1), 19-52. [Google Scholor]
- Ramesh, C., Mohan Kumar, K., Latha, N., & Ragunathan, V. (2012). Green synthesis of Cr2O3 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Escherichia coli. Current Nanoscience, 8(4), 603-607. [Google Scholor]
- Satgurunathan, T., Bhavan, P. S., & Joy, R. D. S. (2018). Green Synthesis of Chromium Nanoparticles and Their Effects on the Growth of the Prawn Macrobrachium rosenbergii Post-larvae. Biological Trace Element Research, 1-10.[Google Scholor]
- Sone, B. T., Manikandan, E., Gurib-Fakim, A., & Maaza, M. (2016). Single-phase α-Cr2O3 nanoparticles’ green synthesis using Callistemon viminalis’ red flower extract. Green Chemistry Letters and Reviews, 9(2), 85-90.[Google Scholor]
- Gupta, N., & Resmi, S. P. (2016). Synthesis of Chromium (V) Oxide Nanoparticles by Mukia Maderaspatana and Mulberry Leaves Extract and Its Characterization. Imperial Journal of Interdisciplinary Research, 2(11), 2454-1362.[Google Scholor]
- Ramesh, C., Mohan Kumar, K., Latha, N., & Ragunathan, V. (2012). Green synthesis of Cr2O3 nanoparticles using Tridax procumbens leaf extract and its antibacterial activity on Escherichia coli. Current Nanoscience, 8(4), 603-607. [Google Scholor]
- Balouria, V., Singh, A., Debnath, A. K., Mahajan, A., Bedi, R. K., Aswal, D. K., Gupta, S. K. (2012). Synthesis and Characterization of Sol-Gel Derived Cr2O3 Nanoparticles. AIP Conf. Proc, 1447, 341-342. [Google Scholor]
- Bhatia, S. (2016). Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications. In Natural polymer drug delivery systems (pp. 33-93). Springer, Cham. [Google Scholor]