Open Journal of Chemistry
Vol. 2 (2019), Issue 1, pp. 11 – 15
ISSN: 2618-0758 (Online) 2618-074X (Print)
DOI: 10.30538/psrp-ojc2019.0008
ISSN: 2618-0758 (Online) 2618-074X (Print)
DOI: 10.30538/psrp-ojc2019.0008
Graphene oxide synthesis by facile method and its characterization
Farman ullah Khan, Sajid Mahmood, Zaheer Ahmad\(^1\), Tariq Mahmood, Zamir Ahmad Nizami
Department of Chemistry, University of Wah, Wah Cantt 47040, Pakistan.; (F.U.K & Z.A)
Department of Chemistry , Division of Science and Technology, University of Education, Lahore 54000, Pakistan.; (S.M)
Nano Sciences and Technology Department, National Centre for Physics, QAU, Islamabad 45320, Pakistan.; (T.M)
Department of Chemistry, University of Sargodha,Sub Campus Bhakkar, Pakistan.; (Z.A.N)
\(^{1}\)Corresponding Author: dr.zaheer.ahmad@uow.edu.pk; Tell: +92-3322569362
Department of Chemistry, University of Wah, Wah Cantt 47040, Pakistan.; (F.U.K & Z.A)
Department of Chemistry , Division of Science and Technology, University of Education, Lahore 54000, Pakistan.; (S.M)
Nano Sciences and Technology Department, National Centre for Physics, QAU, Islamabad 45320, Pakistan.; (T.M)
Department of Chemistry, University of Sargodha,Sub Campus Bhakkar, Pakistan.; (Z.A.N)
\(^{1}\)Corresponding Author: dr.zaheer.ahmad@uow.edu.pk; Tell: +92-3322569362
Copyright © 2019 Farman ullah Khan, Sajid Mahmood, Zaheer Ahmad, Tariq Mahmood, Zamir Ahmad Nizami. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: March 10, 2019 – Accepted: May 21, 2019 – Published: June 30, 2019
Abstract
The graphene and graphene oxide are latest and advanced materials with wide applications in environment, medical applications, industries, defense applications. We have synthesized graphene oxide from graphite flakes by modifying Hummer method in which we used NaNO\(_{2}\) instead of NaNO\(_{3}\). Then we characterized our samples with X-Ray Diffractometry (XRD), Scanning-Electron Microscopy (SEM) and Fourier-Transform Infrared-Spectroscopy (FT-IR). These results confirmed the formation of graphene oxide also through this process. This graphene oxide can further be used for future applications in wastewater treatment and biomedical applications.
Keywords:
Graphene oxide, nanochemistry.
1. Introduction
The importance of graphene and its composites is increasing due to their enlarged applications in the solar cells, catalysis and hydrogen storage. They are used in sensors, nanoelectronics and nanocomposites [1, 2, 3, 4, 5]. Graphene is the single layer of graphite. All the carbon atoms of graphene are sp\({}^{2}\)hybridized. They are arranged in a honey comb lattice like structure. Graphene is carbon allotrope. It has hexagonal atomic structure and therefore hexagonal nanoparticles are easily adjusted in their layers. Their hydrogels have crosslinked nanoshheet structure [6]. The nanocomposite of TiO2 and graphene is exhibiting large surface area, higher activity and the N\({}_{2}\) selection and at low temperature high resistance towards water and sulfur dioxide and also, high redox activity by which the catalytic reaction is favored [7]. Several studies have shown that metal-metal oxide-graphene have triple junction structure formation. They act like the electrocatalyst for the oxygen reduction for the applications in the PEM fuel cell. The tests performed showed that the durability of Pt became better than Pt on graphene sheets but also from Pt electrocatalysts which are supported on Carbon material, eg; Carbon nano-tubes [8]. Aijun etal., have worked on graphene and g-C3N4 interface by the state of art of the hybrid functional DFTprocedure and incorporated the long range dispersion correction. They for first time revealed that electronic coupling is strong at graphene and g-C3N4 interface [9]. Xuefeng etal; reviewed the most recent research on the graphene materials and their antimicrobial activities. They also discussed the physiochemical properties of graphene materials, their experimental surrounding and selected mocroorganisms and the interaction between them to further explore the controversial antimicrobial properties [10]. Wang etal; prepared metal oxide and graphene nanocomposite. They studied the properties of various metal oxide and graphene nanocomposite for the super capacitors and also for electrochemical catalysis [11]. Vats etal; synthesized nanocomposite of pristine-graphene with the palladium. They used swollen liquids crystal as the soft template. They observed that the catalytic activity of the nanocomposite is better than the Pd-RGO nanocomposite in one of the hydrogenation reaction of nitro-phenol and microwave assisted carbon-carbon coupling reaction which was sixteen times higher [12]. Kian etal; studied the graphene and the molecules like graphene for their role in the solar cells [13]. Mousavi etal; prepared PV-RGO and used as anodic battery material. That composite showed high areal, volumetric and the current density [14]. Meng etal; synthesized nanocomposite of silver nanoparticles decorated with graphene by chemical reduction process with assistance by supercritical CO\({}_{2}\) (ScCO\({}_{2}\)). They studied tribological properties of the nanocomposite. It showed the high lubricating function of the graphene and the roughness of surface of the sliding ball is reduced and prevents direct interaction [15]. Tengfei etal; worked on the synthesis of nanocomposite of iron oxide and silver nanoparticles with graphene. They compared the Ag nanoparticles with this nanocomposite. They found that the nanocomposite was showing enhanced antibacterial activity towards the Gram negative bacteria, E.coli and the Gram positive bacteria which is S. aureus [16]. Li etal; synthesized graphene sheet, polymeric carbon-nitride nanocomposite which work as metal free catalyst for activation of oxygen for the selective oxidation of the sec. carbon-hydrogen bonds of saturated hydrocarbons like cyclohexane. It was observed to be the most stable catalyst and having high chemoselectivity for the sec. C-H bond of different saturated alkane [17]. Liwen etal; synthesized a nanocomposite of Graphene Oxide and Sulfur for the immobilization of sulfur in cathodic material of Li-S cells [18]. Deepak etal; synthesized Graphene Oxide and studied its antibacterial activities [19]. Zheng etal; prepared reduced graphene oxide by reducing graphene oxide by help of a reducing agent (caffeic acid) which was having high carbon-oxygen ratio i.e \eqref{GrindEQ__7_15_} which is best reduced graphene oxide prepared from green reducing ragent [20]. Hongmei etal; used solvothermal method and developed magnetic-reduced graphene oxide (MRGO) nanocomposite. The nanocomposite was having high removal efficiency. It was observed that the M-RGO composite is effective adsorbent used for the removal of dyes pollutant [21]. Myungwoo etal; reviewed the graphene oxide synthesis which was developed through chemical vapor deposition method and studied its application [22]. Zhang etal; developed reduced graphene oxide and NiO nanocomposite which was used for the absobtion of the chromium ion (Cr (VI)). The composite showed maximum adsoption capacity of chromium ion atpH=4 and T= 25 \({}^{0}\)c which was higher than any other reported so far [23]. Marcano etal; prepared graphene oxide by modification in the Hummer method as they excluded NaNO\({}_{3}\). They increased KMnO\({}_{4}\) and used H\({}_{3}\)PO\({}_{4\ }\)and H\({}_{2}\)SO\({}_{4}\) mixture in the ratio of 1:9 which showed improvement in the oxidation process of graphene [24].2. Method and Materials
We took 2g of graphite and NaNO\({}_{2}\) and mixed in about 100mL of H\({}_{2}\)SO\({}_{4}\) (conc.) in 1000mL volumetric flask and put in ice-bath. The mixture was continuously stirred on hot plate for about 1 hour and then added 6 g of KMNO\({}_{4}\) slowly. Then removed ice bath and kept the mixture under stirring for 2 days. About 90mL of distilled water was added to this and brown color appeared. The solution was stirred continuously. Then 15mL of hydrogen peroxide\({}_{\ }\)was added to this solution and yellow color appeared. We washed this mixture with 10\% HCl. Then centrifuged at 5000 rpm for 5 minute. This filtrate was decanted. The remaining (graphene oxide) was dried at 110\({}^{0}\)C and then calcined for 3 hours at 550\({}^{0}\)C in muffle furnce. The graphene oxide thus obtained was grind and characterized for further analysis.3. Results and Discussions
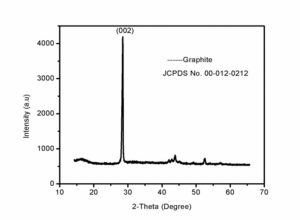
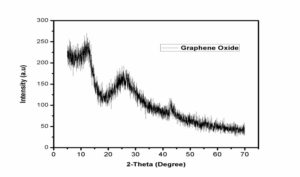
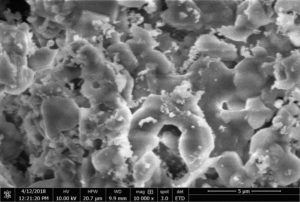
For the characterization of prepared nanoparticles XRD is one of the best technique. It characterizes the purity andphase of the nanomaterial. XRD gives detail of diffraction angle, the interlayer spacing and mainly the crystallite size. The XRD used in our work was Bruker D8 advance. Fig.1 shows XRD patern of graphite used in this work. Fig.2 is showing wide-angle XRD peak of our prepared graphene oxide. In graphene oxide XRD spectra, the major peak at 11.6\({}^{0}\) and two weak peaks at 2\(\theta\)= 43\({}^{0}\) and 2\(\theta\)= 45\({}^{0}\) clearly describe the graphite structure [22, 25]. We characterized the graphene oxide by the Field-emission scanning-electron microscopy (FESEM) for their surface morphology. Fig.3 shows FESEM image of graphene oxide at different magnifications. It is clear from the FESEM image of GO that their layers are smooth in appearance [1]25}. The prepared graphene oxide was characterized by FT-IR for functional-groups checking. Figure. 4 show the FT-IR spectra of the graphene oxide. The major peak at about 3500 cm\({}^{-1}\)\({}_{\ }\)shows the presence of hydroxyl (OH) groups [25]. Some peaks were also observed in fingerprint region at 750cm\({}^{-1\ }\)- 600cm\({}^{-1}\) which may be the bending vibrations due to C-O, and C-O-C bending vibrations.4. Conclusion
In summary, we synthesized Graphene Oxide from graphite powder by using NaNO\({}_{2}\) instead of NaNO\({}_{3.\ }\)The resultant product obtained was characterized through different analytical techniques like, XRD, FESEM, FT-IR. The obtained result was matched with literature and this was confirmed that the graphene oxide has formed. The oxidation of graphene can be increased by using NaNO\({}_{2.\ }\)This can be used for different purposes specially as adsorbant of heavy metals.Figure 1. XRD pattern of graphite
Figure 2. XRD pattern of graphene oxide
Figure 3. SEM image of Graphene Oxide
Figure 4. FT-IR spectra of Graphene oxide
Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
The authors declare no conflict of interest.References
- Chen, H., & Carroll, K. C. (2016). Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene. Environmental pollution, 215, 96-102.[Google Scholor]
- Cho, K. Y., Seo, H. Y., Yeom, Y. S., Kumar, P., Lee, A. S., Baek, K. Y., & Yoon, H. G. (2016). Stable 2D-structured supports incorporating ionic block copolymer-wrapped carbon nanotubes with graphene oxide toward compact decoration of metal nanoparticles and high-performance nano-catalysis. Carbon, 105, 340-352.[Google Scholor]
- Kwon, S. N., Jung, C. H., & Na, S. I. (2016). Electron-beam-induced reduced graphene oxide as an alternative hole-transporting interfacial layer for high-performance and reliable polymer solar cells. Organic Electronics, 34, 67-74. [Google Scholor]
- Xia, G., Tan, Y., Wu, F., Fang, F., Sun, D., Guo, Z., & Yu, X. (2016). Graphene-wrapped reversible reaction for advanced hydrogen storage. Nano Energy, 26, 488-495.[Google Scholor]
- Park, C. S., Yoon, H., & Kwon, O. S. (2016). Graphene-based nanoelectronic biosensors. Journal of Industrial and Engineering Chemistry, 38, 13-22. [Google Scholor]
- Jiao, T., Guo, H., Zhang, Q., Peng, Q., Tang, Y., Yan, X., & Li, B. (2015). Reduced graphene oxide-based silver nanoparticle-containing composite hydrogel as highly efficient dye catalysts for wastewater treatment. Scientific reports, 5, 11873.[Google Scholor]
- Lu, X., Song, C., Chang, C. C., Teng, Y., Tong, Z., & Tang, X. (2014). Manganese Oxides Supported on TiO2-Graphene Nanocomposite Catalysts for Selective Catalytic Reduction of NO X with NH3 at Low Temperature. Industrial & Engineering Chemistry Research, 53(29), 11601-11610.[Google Scholor]
- Kou, R., Shao, Y., Mei, D., Nie, Z., Wang, D., Wang, C., ... & Wang, Y. (2011). Stabilization of electrocatalytic metal nanoparticles at metal-metal oxide-graphene triple junction points. Journal of the American Chemical Society, 133(8), 2541-2547.[Google Scholor]
- Du, A., Sanvito, S., Li, Z., Wang, D., Jiao, Y., Liao, T., ... & Smith, S. C. (2012). Hybrid graphene and graphitic carbon nitride nanocomposite: gap opening, electron-hole puddle, interfacial charge transfer, and enhanced visible light response. Journal of the American Chemical Society, 134(9), 4393-4397.[Google Scholor]
- Zou, X., Zhang, L., Wang, Z., & Luo, Y. (2016). Mechanisms of the antimicrobial activities of graphene materials. Journal of the American Chemical Society, 138(7), 2064-2077. [Google Scholor]
- Wang, D., Kou, R., Choi, D., Yang, Z., Nie, Z., Li, J., ... & Liu, J. (2010). Ternary self-assembly of ordered metal oxide-graphene nanocomposites for electrochemical energy storage. ACS nano, 4(3), 1587-1595. [Google Scholor]
- Vats, T., Dutt, S., Kumar, R., & Siril, P. F. (2016). Facile synthesis of pristine graphene-palladium nanocomposites with extraordinary catalytic activities using swollen liquid crystals. Scientific reports, 6, 33053.[Google Scholor]
- Loh, K. P., Tong, S. W., & Wu, J. (2016). Graphene and graphene-like molecules: prospects in solar cells. Journal of the American Chemical Society, 138(4), 1095-1102.[Google Scholor]
- Beladi-Mousavi, S. M., Sadaf, S., Mahmood, A. M., & Walder, L. (2017). High Performance Poly (viologen)-Graphene Nanocomposite Battery Materials with Puff Paste Architecture. ACS nano, 11(9), 8730-8740. [Google Scholor]
- Meng, Y., Su, F., & Chen, Y. (2016). Supercritical fluid synthesis and tribological applications of silver nanoparticle-decorated graphene in engine oil nanofluid. Scientific reports, 6, 31246.[Google Scholor]
- Tian, T., Shi, X., Cheng, L., Luo, Y., Dong, Z., Gong, H., ... & Liu, Z. (2014). Graphene-based nanocomposite as an effective, multifunctional, and recyclable antibacterial agent. ACS applied materials & interfaces, 6(11), 8542-8548. [Google Scholor]
- Li, X. H., Chen, J. S., Wang, X., Sun, J., & Antonietti, M. (2011). Metal-free activation of dioxygen by graphene/g-C3N4 nanocomposites: functional dyads for selective oxidation of saturated hydrocarbons. Journal of the American Chemical Society, 133(21), 8074-8077. [Google Scholor]
- Ji, L., Rao, M., Zheng, H., Zhang, L., Li, Y., Duan, W., ... & Zhang, Y. (2011). Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. Journal of the American Chemical Society, 133(46), 18522-18525. [Google Scholor]
- Gupta, D. K., Rajaura, R. S., & Sharma, K. (2015). Synthesis and Characterization of Graphene Oxide Nanoparticles and their Antibacterial Activity. Suresh Gyan Vihar University International Journal of Environment, Science and Technology, 1(1), 16-24. [Google Scholor]
- Bo, Z., Shuai, X., Mao, S., Yang, H., Qian, J., Chen, J., ... & Cen, K. (2014). Green preparation of reduced graphene oxide for sensing and energy storage applications. Scientific reports, 4, 4684. [Google Scholor]
- Sun, H., Cao, L., & Lu, L. (2011). Magnetite/reduced graphene oxide nanocomposites: one step solvothermal synthesis and use as a novel platform for removal of dye pollutants. Nano Research, 4(6), 550-562. [Google Scholor]
- Son, M., & Ham, M. H. (2017). Low-temperature synthesis of graphene by chemical vapor deposition and its applications. FlatChem, 5, 40-49.[Google Scholor]
- Zhang, K., Li, H., Xu, X., \& Yu, H. (2018). Synthesis of reduced graphene oxide/NiO nanocomposites for the removal of Cr (VI) from aqueous water by adsorption. Microporous and Mesoporous Materials, 255, 7-14.[Google Scholor]
- Marcano, D. C., Kosynkin, D. V., Berlin, J. M., Sinitskii, A., Sun, Z., Slesarev, A., ... & Tour, J. M. (2010). Improved synthesis of graphene oxide. ACS nano, 4(8), 4806-4814.[Google Scholor]
- Atchudan, R., Edison, T. N. J. I., Perumal, S., Karthikeyan, D., & Lee, Y. R. (2016). Facile synthesis of zinc oxide nanoparticles decorated graphene oxide composite via simple solvothermal route and their photocatalytic activity on methylene blue degradation. Journal of Photochemistry and Photobiology B: Biology, 162, 500-510.[Google Scholor]