Trends in Clinical and Medical Sciences
ISSN: 2791-0814 (online) 2791-0806 (Print)
DOI: 10.30538/psrp-tmcs2023.0039
Growth pattern in thalassemic children and their correlation with serum ferritin level in a transfusion dependent thalassemic children on oral chelation therapy
Gautam Medhi\(^1\), Amit Bhattacharjee\(^1\), Diganta Barman\(^1\), Mahibur Rahman\(^{1,*}\), Sabir Hussain\(^1\) and Dorothy Hazowary\(^1\)
\(^{1}\) Department of Pediatrics, Gauhati Medical College and Hospital, Guwahati, Assam, India.
Correspondence should be addressed to Mahibur Rahman at rahman.mahibur786@gmail.com; Tell: – 9435365872
Abstract
Aims and objectives: To study the growth pattern in thalassemic children who are on regular chelation therapy and blood transfusion in comparison to normal children and serum ferritin level in these children and its relation to growth pattern and oral chelation therapy.
Methods: The present study was a hospital-based cross-sectional study. One hundred and one transfusion-dependent thalassemic children on oral chelation therapy, attending the pediatric ward in Gauhati Medical College and Hospital, Guwahati, Assam, were enrolled during the study period from 1st May 2021 to 30th April 2022.
Results: This study provides evidence that children suffering from transfusion-dependent thalassemia (TDT) are prone to growth retardation, with underweight, stunting, and wasting being 31.6%, 52.4%, and 27.7%, respectively.
Conclusion: In conclusion, short stature is an important complication in TDT. Growth in patients with TDT is significantly related to age, sex, hemoglobin level, and iron overload status. Management with hypertransfusion and good control of iron overload is important to achieve optimum growth in patients with TDT.
Keywords:
1. Introduction
Beta-thalassemia syndromes are a group of genetic blood disorders characterized by reduced or incomplete beta globin chain synthesis, leading to lower hemoglobin levels in red blood cells (RBC), lower RBC output, and anemia [1]. The harmful effects of excess globin chain subunits can be attributed to the pathophysiology of thalassemias. Excess chains affect red cell precursors and red cells, leading to erythropoietic marrow enlargement that is ineffective in creating mature red cells, severely impairing development, bone formation, and growth. Iron deposition on the endocrine organs, liver, and heart, which occurs from increased intestine absorption and the consequences of blood transfusion, is the primary cause of morbidity and mortality [2].
According to the Census of India 2011, the average prevalence of \(\beta\) thalassemia carriers is 3-4%, which equates to 35 to 45 million carriers in the multi-ethnic, culturally, and linguistically diversified population of 1.21 billion people, including roughly 8% of tribal communities [3,4,5]. Diagnosis of thalassemias requires a combination of laboratory tests, including automatic hematology analyzer evaluation of red blood cell indices, Hb analysis, and quantification of Hb A2 and Hb F. The thalassemic disorders and carriers can be distinguished using high-performance liquid chromatography (HPLC) and capillary zone electrophoresis (CE) system [6].
Patients with thalassemia major (TM) typically exhibit delayed puberty and development, along with a decrease in ultimate height. Despite significant advancements in therapy, growth failure in TM has long been recognized. Up to the age of 9 to 10 years, the child with TM has a specific development pattern that is quite typical; after this age, a slowdown of growth velocity and a diminished or non-existent pubertal growth surge are noticed. The fusing of the growth plates often takes place at the end of the second decade of life [7, 8].
The causes of growth failure are complex and multifaceted, mainly due to chronic anemia, hypoxia, chronic liver disease, zinc and folic acid deficiency, iron overload, intensive use of chelating agents, emotional factors, endocrinopathies, and GH-IGF-1 axis dysregulation [8]. Early-life high serum ferritin levels are linked to eventual short stature, suggesting that iron chelation treatment can prevent or reduce this issue [8,9,10].
This study aims to investigate two important aspects related to growth patterns in thalassemic children:- The growth pattern in transfusion-dependent thalassemic children on regular chelation therapy and blood transfusion in comparison to normal children.
- The relationship between serum ferritin levels in these children, their growth pattern, and oral chelation therapy.
2. Methods
The present study was a hospital-based cross-sectional study in which 101 transfusion-dependent thalassemic children on oral chelation therapy, attending the Pediatric Ward in Gauhati Medical College and Hospital, Guwahati, Assam, were enrolled during the study period from 1st May 2021 to 30th April 2022.2.1. Inclusion criteria
Children diagnosed with thalassemia major and intermedia based on HPLC report and on oral chelation therapy if serum ferritin is above 1000 \(\mu\)g/L.2.2. Exclusion criteria
- Children who are having severe systemic illness.
- Children who are not taking blood transfusion and not on oral chelation therapy despite serum ferritin level above 1000 \(\mu\)g/L.
- Thalassemia minor.
2.3. Study population
All children aged 1-13 years old with transfusion-dependent thalassemia (TDT).2.4. Sample size and sampling
Sample size was calculated and came out to be 116. A sample of 101 transfusion-dependent thalassemia children were selected by consecutive sampling from the Pediatrics Ward.2.5. Study tools
- Semi-structured proforma
- Anthropometry measurements
2.6. Method of data collection
A predesigned proforma was used to collect data and information. Written informed consent was obtained from parents/guardians for enrollment of their children in the study. Detailed anthropometric measurements were taken, and grading of malnutrition was done. All cases were evaluated using the following variables: age, sex, type of thalassemia, age at diagnosis, weight, length/height, MUAC, Z-scores calculated for weight for age, height/length for age, and weight for height/length, BMI. Clinical examinations were done followed by routine investigations.2.7. Ethical issues
Approval from the institutional ethical committee was obtained before conducting the study. Informed written consent was obtained from all the participants. Confidentiality was maintained.2.8. Statistical analysis
Statistical analyses were performed using SPSS version 20. Results were expressed as mean \(\pm\) standard deviation for continuous variables and as number (%) for categorical data. Linear regression, linear (Pearson) correlation, Spearman nonparametric correlation were applied where applicable. A p-value less than 0.05 was considered significant.3. Results
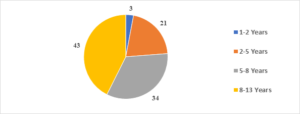
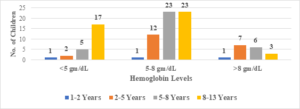
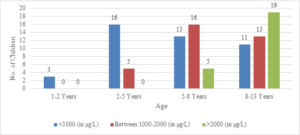
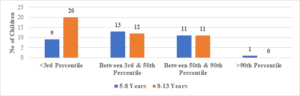
In this study, the average age of the participants was found to be 7.6\(\pm\)2.6 years, with the youngest participant being 1 year old and the oldest being 13 years old (Figure 1). Among the 101 transfusion-dependent thalassemic children, the majority (70.2%) were male. The average age at diagnosis was 22.7\(\pm\)10.1 months, with the youngest case being diagnosed at 7 months and the oldest at 44 months. The average pre-transfusion Hb level was 6.3\(\pm\)1.5 gm/dL (Figure 2). Out of the 101 children in the study, 24 (23.7%) had serum ferritin levels greater than 2000 \(\mu\)g/L, and the majority of them (79.1%) were over 8 years old. In contrast, 43 (42.5%) children had serum ferritin levels below 1000 \(\mu\)g/L, and only 11 (25.5%) of them were over 8 years old (Figure 3). Of the 101 children in the study, 32 (31.6%) were underweight according to WHO and IAP charts by age. Of these, 20 (46.5%) were over 8 years old. The percentage of underweight children was 26.4%, 14.2%, and 0% for age groups 5-8 years, 2-5 years, and under 2 years old, respectively (Figures 4 and 5). Among the 101 transfusion-dependent thalassemic children, 53 (52.4%) were stunted according to WHO and IAP charts by age. Of these, 33 (76.7%) were over 8 years old. The percentage of stunted children was 44.1%, 23.8%, and 0% for age groups 5-8 years, 2-5 years, and under 2 years old, respectively. Among the 24 transfusion-dependent thalassemic children in the age group of 1-5 years in our study, 3 (12.5%) were found to be wasted, and all three belonged to the age group of 2-5 years old. None of them were below 2 years old.
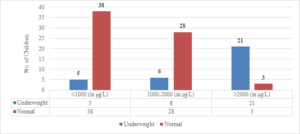
Out of the 24 children with serum ferritin levels above 2000 \(\mu\)g/L, 21 (87.5%) were found to be underweight. Of the 34 children with serum ferritin levels between 1000-2000 \(\mu\)g/L, 6 (17.6%) were underweight. Only 5 (11.6%) children out of 43 whose serum ferritin levels were below 1000 \(\mu\)g/L were underweight (Figure 6). The correlation coefficient (r) was found to be 0.6899, with a 95% confidence interval of 0.5715 to 0.7802. The two-tailed P value was less than 0.0001, which was considered extremely significant.
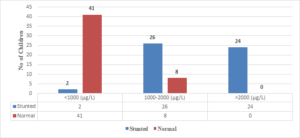
In this study, all 24 children with Serum Ferritin Levels above 2000 \(\mu\)g/L were found to be stunted, while 26 (76.4%) out of 34 children with levels between 1000-2000 \(\mu\)g/L were stunted. Only 2 (4.6%) out of 43 children with Serum Ferritin Levels below 1000 \(\mu\)g/L were stunted (Figure 7). The correlation coefficient (r) is 0.802, with a 95% confidence interval of 0.7179 to 0.8615, and the two-tailed P value is < 0.0001, which is considered extremely significant.
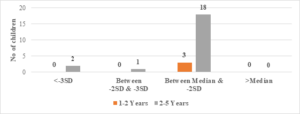
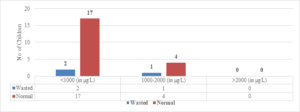
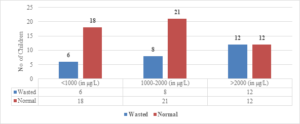
Regarding wasted children, 12 (50%) out of 24 children with Serum Ferritin Levels above 2000 \(\mu\)g/L were found to be wasted, while 8 (27.5%) out of 29 children with levels between 1000-2000 \(\mu\)g/L were wasted. Only 6 (25%) out of 24 children with Serum Ferritin Levels below 1000 \(\mu\)g/L were wasted (Figure 9). The two-tailed P value is 0.2881, which is considered not significant. For children with Serum Ferritin Levels between 1000-2000 \(\mu\)g/L, 1 (20%) out of 5 children was wasted, while only 2 (10.5%) out of 19 children with levels below 1000 \(\mu\)g/L were wasted. None of the children below 5 years of age had Serum Ferritin Levels above 2000 \(\mu\)g/L (Figure 8). The two-tailed P value is 0.0184, which is considered significant with a 95% confidence interval of 0.09117 to 0.7384.
In terms of Mid Upper Arm Circumference (MUAC), only 1 child with Serum Ferritin Level below 1000 \(\mu\)g/L had MUAC below 11.5 cm, while no other child had MUAC below this level. The correlation coefficient (r) is -0.046, with a 95% confidence interval of -0.4485 to 0.3564, and the two-tailed P value is 0.7987, which is considered not significant.
Figure 1. Age distribution
Figure 2. Pre-transfusion hemoglobin level distribution
Figure 3. Serum ferritin level distribution according to age
FIgure 4. Weight for age: 1-5 years of age (compared with WHO chart)
Figure 5. Weight for age: >5 years (compared with IAP chart)
Figure 6. Correlation of S. ferritin with weight for age
Figure 7. Correlation of S. ferritin with length/height for age
Figure 8. Correlation of S. ferritin with BMI
Figure 9. Correlation of S. ferritin with weight for height
4. Discussion
The mean pre-transfusion hemoglobin level in this study was 6.3\(\pm\)1.5 gm/dL, which is well below the recommended level of 10 gm% [11] for adequate growth of thalassemic children. 83.2% of the children in the study had pre-transfusion hemoglobin levels of less than 8 gm%. Similar results were found in other studies, such as those conducted by Pemde et al. in New Delhi, India [12] (9.21 g/dL), and Nokeaingtong et al. in Chiang Mai University Hospital, Thailand [13](7.4\(\pm\)1.0 g/dL).
Overall, 31.6% of the children in this study were underweight, with underweight being more prevalent with increasing age. There was a strong statistical correlation between underweight and increasing age, which is consistent with observations in other studies, such as those conducted by Moiz et al. in Karachi, Pakistan [14] (40%), Rathaur et al. in Uttarakhand, India [15] (77%), and Chhabra and Sodhi in Punjab, India [16] (78.1%).
In this study, 52.4% of the children were found to be stunted, with stunting being more prevalent in patients over the age of 8 years and approximately half of those between 5-8 years. Therefore, stunting was found to be more prevalent with increasing age. Similar observations were seen in studies conducted by Nokeaingtong et al. in Chiang Mai University Hospital, Thailand [13] (38%) and Moiz et al. in Karachi, Pakistan [14] (65.4%).
Wasting was found in 27.7% of the children in this study, with similar prevalence between the age groups of 5-8 years and 8-13 years. This observation is consistent with those of other studies, such as those conducted by Moiz et al. in Karachi, Pakistan [14] (42%) and Pemde et al. in New Delhi, India [12] (24.1%).
The present study found that 96% of the children between 1-5 years had MUAC within the normal range, indicating adequate nutrition. Among children with serum ferritin levels above 2000 \(\mu\)g/L, 87.5% were underweight, 100% were stunted, and none below the age of 5 years had serum ferritin levels above 2000. Furthermore, 50% of those over the age of 5 years were wasted. However, no correlation was established between serum ferritin and MUAC in this study.
Regarding children with serum ferritin levels below 1000 \(\mu\)g/L, 11.6% were underweight, 4.6% were stunted, and 10.5% were wasted below the age of 5 years, while 25% were wasted above the age of 5 years. The present study suggests a correlation between high serum ferritin levels and growth retardation, which is consistent with observations in similar studies, such as those conducted by Rathaur et al. in Uttarakhand, India [15], 2020, and Hala Saad Bash et al. in the University of Babylon, Iraq [17], 2021 concluded the same.
5. Conclusion
This study provides compelling evidence that children with transfusion-dependent thalassemia (TDT) are highly susceptible to growth retardation. The study found that 31.6% of children with TDT were underweight, 52.4% were stunted, and 27.7% were wasting. The findings suggest that short stature is a critical complication in TDT and that growth in TDT patients is significantly related to age, sex, hemoglobin level, and iron overload status. Effective management of TDT with hypertransfusion and optimal control of iron overload is crucial to achieving optimal growth in TDT patients.6. Limitations
This study has several limitations that need to be addressed. Firstly, the study did not collect information on pubertal development, making it impossible to determine the causal relationship between short stature and delayed puberty. Secondly, the study did not investigate other potential factors that may contribute to growth retardation in TDT patients, such as the bony side effects of deferoxamine or nutritional deficiencies. The results of this study are therefore limited to the effects of anemia and iron overload on short stature in TDT patients.Author Contributions:
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.Conflicts of Interest:
"The authors declare no conflict of interest."References
- Galanello, R., & Origa, R. (2010). Beta-thalassemia. Orphanet Journal of Rare Diseases, 5, 11.[Google Scholor]
- Borgna-Pignatti, C., & Galanello, R. (2009). Thalassemias and Related Disorders: Quantitative Disorders of Hemoglobin Synthesis (12th ed.). Philadelphia, Pa, USA Lippincott Williams and Wilkins.[Google Scholor]
- Colah, R. B., & Gorakshakar, A. (2014). Control of thalassemia in India. Thalassemia Reports, 4(2), 84-90.[Google Scholor]
- Madan, N., Sharma, S., Sood, S., Colah, R., & Bhatia, H. (2010). Frequency of \(\beta\)-thalassemia trait and other hemoglobinopathies in northern and western India. Indian Journal of Human Genetics, 16(1), 16-25.[Google Scholor]
- Colah, R., Italia, K., & Gorakshakar, A. (2017). Burden of thalassemia in India: The road map for control. Pediatric Hematology Oncology Journal, 2(4), 79-84.[Google Scholor]
- Munkongdee, T., Chen, P., Winichagoon, P., Fucharoen, S., & Paiboonsukwong, K. (2020). Update in laboratory diagnosis of thalassemia. Frontiers in Molecular Biosciences, 7, 74. [Google Scholor]
- Rodda, C. P., Reid, E. D., Johnson, S., Doery, J., Matthews, R., & Bowden, D. K. (1995). Short stature in homozygous beta-thalassaemia is due to disproportionate truncal shortening. Clinical Endocrinology, 42(6), 587-92.[Google Scholor]
- Kyriakou, A., & Skordis, N. (2009). Thalassaemia and aberrations of growth and puberty. Mediterranean Journal of Hematology and Infectious Diseases, 1(1), e2009003. [Google Scholor]
- Shalitin, S., Carmi, D., Weintrob, N., Phillip, M., Miskin, H., Kornreich, L., et al. (2005). Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. European Journal of Haematology, 74(2), 93-100.[Google Scholor]
- Garc\i a-Mayor, R. V., Olivie, A., Catalina, F., Castro, M., Iraeta, R., & Reparaz, A. (1993). Linear growth in thalassemic children treated with intensive chelation therapy. Hormone Research, 40(5-6), 189-193.[Google Scholor]
- National Health Portal. (n.d.). Thalassemia. Retrieved from \url{https://www.nhp.gov.in/thalassemia\_pg}
- Pemde, H., Chandra, J., Singh, V., Gupta, D., Sharma, R., & Dutta, A. K. (2011). Physical growth in children with transfusion-dependent thalassemia. Pediatric Health, Medicine and Therapeutics, 13, 57-61.[Google Scholor]
- Nokeaingtong, K., Charoenkwan, P., Silvilairat, S., Saekho, S., Pongprot, Y., & Dejkhamron, P. (2016). A longitudinal study of growth and relation with anemia and iron overload in pediatric patients with transfusion-dependent thalassemia. Journal of Pediatric Hematology/Oncology, 38(1), 42-47.[Google Scholor]
- Moiz, B., Habib, A., Sawani, S., Raheem, A., Hasan, B., & Gangwani, M. (2018). Anthropometric measurements in children having transfusion-dependent beta thalassemia. Hematology, 23(4), 248-252.[Google Scholor]
- Rathaur, V., Imran, A., & Pathania, M. (2020). Growth pattern in thalassemic children and their correlation with serum ferritin. Journal of Family Medicine and Primary Care, 9(2), 1166-1169.[Google Scholor]
- Chhabra, G. S., & Sodhi, M. K. (2016). Pattern of growth retardation and sexual maturation in children having beta thalassaemia. Journal of Nepal Paediatric Society, 36(1), 56-60.[Google Scholor]
- Saad, B. H., Abdul-AM, A. H. H., Hussein, A. M. B., & Mazin, J. M. (2021). The study of serum ferritin level as a predictor of growth retardation in thalassemia-major. Archivos Venezolanos de Farmacologia y Terapeutica, 40(5), 492-501.[Google Scholor]